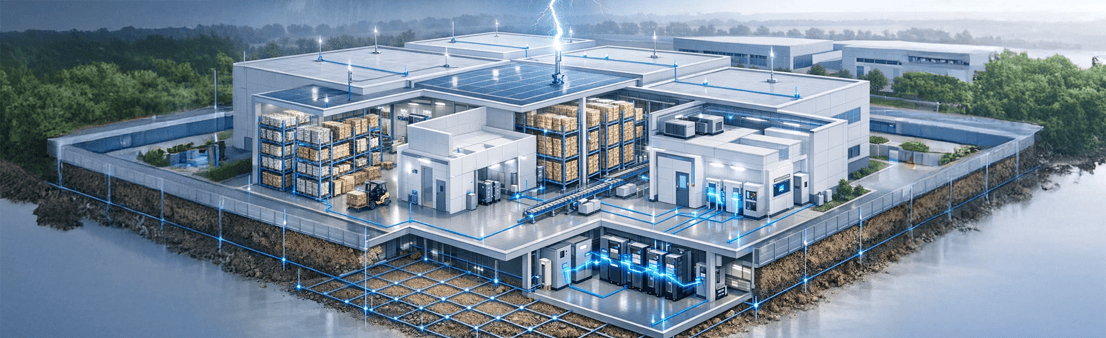
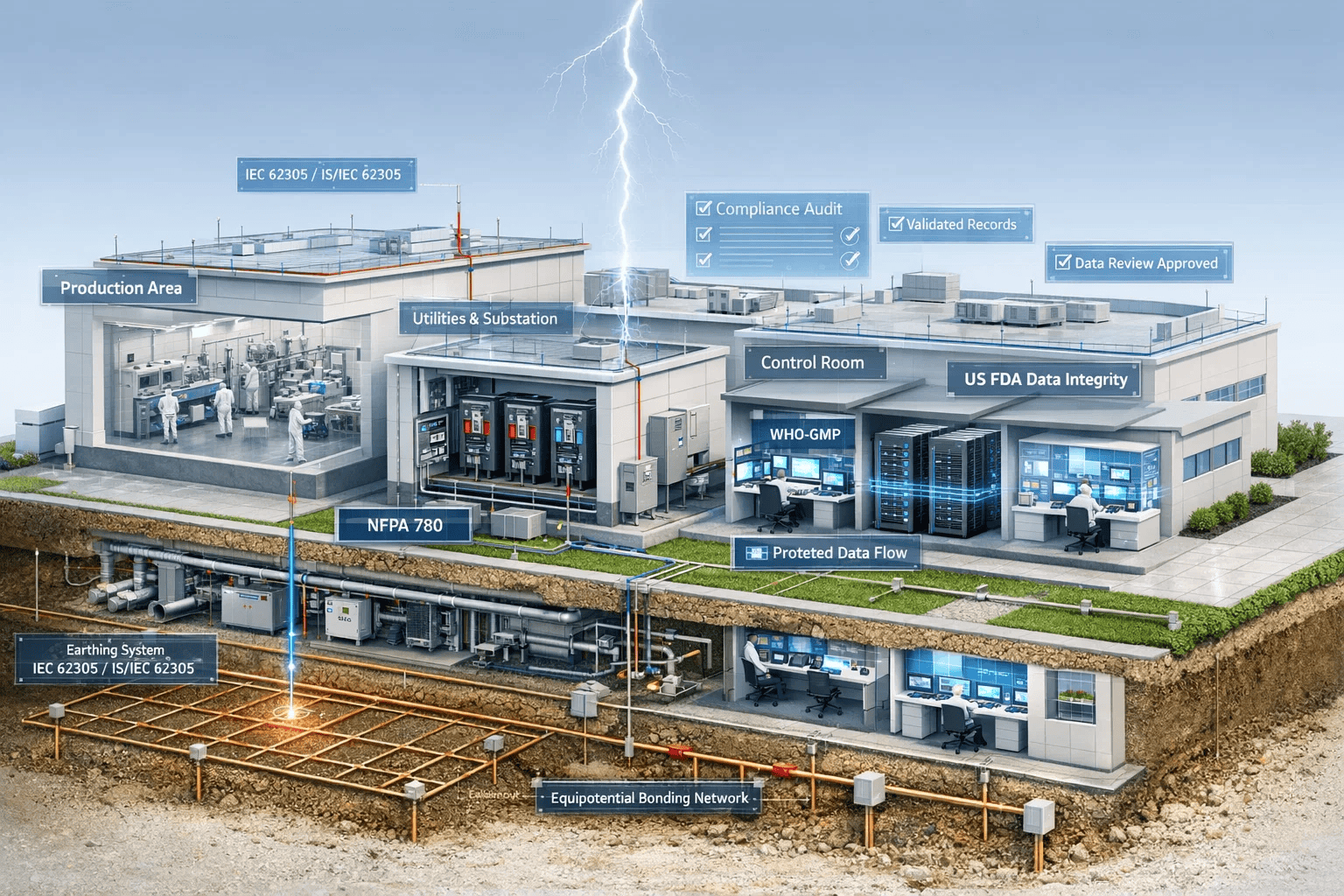
Safeguarding GMP Facilities, Critical Assets, Digital Integrity & Human Life
Lightning protection in the pharmaceutical industries is designed in three integrated layers:
- External Lightning Protection System (LPS)
- Internal Surge Protection Devices (SPDs)
- Earthing, Bonding & Equipotential Grounding
LPES solutions control lightning energy from interception to safe dissipation, mitigating:
- Direct lightning strikes
- Induced over voltages and switching surges
- Ground potential rise
- Electrostatic discharge (ESD)
This ensures uninterrupted, validated pharmaceutical operations and long-term asset protection.
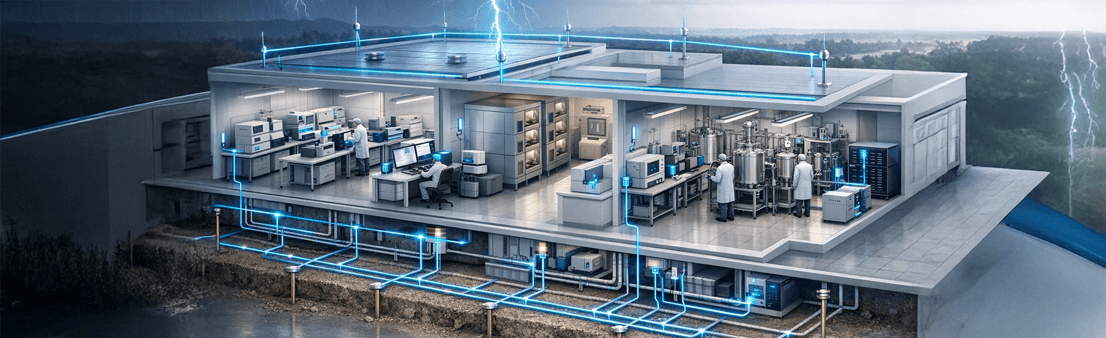
Manufacturing & Production Areas
Pharmaceutical production environments operate under continuous, validated, and contamination-controlled conditions. Even momentary electrical disturbances can invalidate entire batches and disrupt patient supply chains
Protecting Inventory, Compliance & Business Continuity
Pharmaceutical warehouses store high-value APIs, excipients, intermediates, finished products, and temperature-sensitive formulations.
- Raw material warehouses
- API and excipient storage areas
- Finished goods warehouses
- Cold rooms and temperature-controlled storage
- Roof-mounted lightning arresters
- Earthing and bonding networks
- Surge protection for refrigeration ,lighting, and power systems
- High-value pharma inventory at lightning risk
- Temperature failures cause product loss
- Surges trigger compliance deviations
- Downtime disrupts business continuity
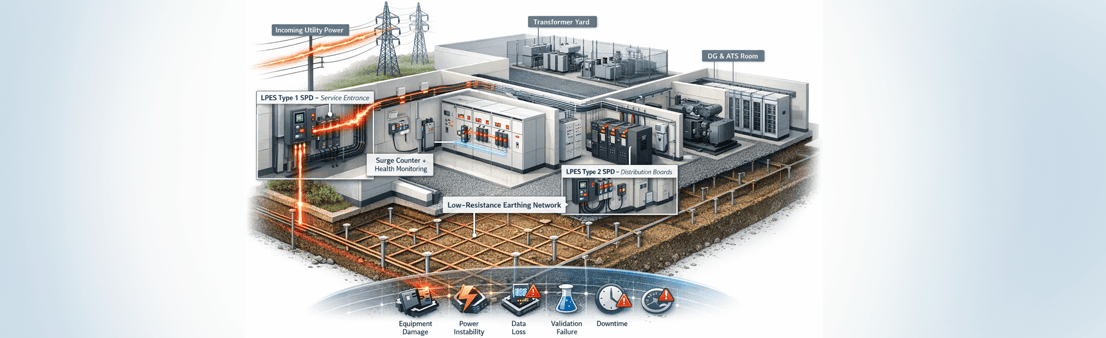
- HT/LT panels
- Transformers and substations
- DG sets and emergency power systems
- UPS rooms and critical power distribution
- Type 1 SPDs at service entrances
- Type 2 SPDs at distribution boards
- Low-resistance earthing systems
- Surge counters and monitoring devices
- Lightning/surges damage stored products
- Surges disrupt lighting & handling
- Refrigeration failure → temperature excursions
- Poor earthing/GPR → safety & compliance risk
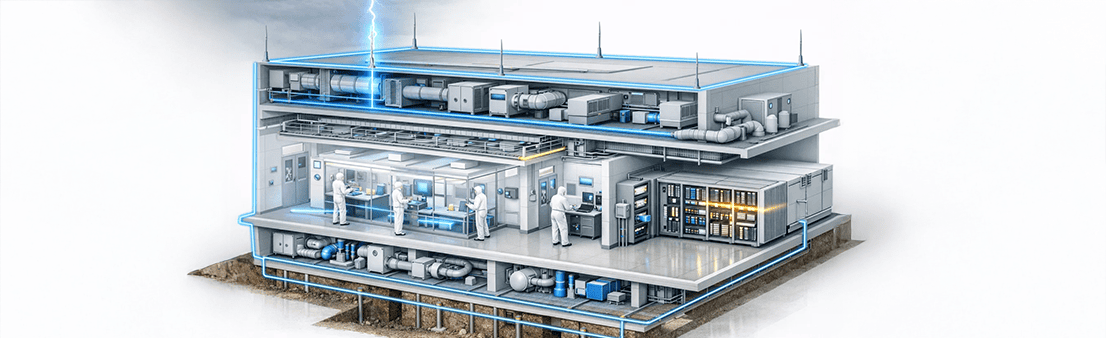
- HVAC systems
- AHUs, chillers, and BMS panels
- Environmental monitoring systems
- Surge protection for HVAC control panels
- Bonding of ducting and metallic structures
- Type 2 & Type 3 SPDs for control electronics
- Lightning & surges disrupt HVAC, AHUs, chillers & BMS
- Over voltages affect control electronics & sensors
- Monitoring failure → undetected GMP excursion
This prevents temperature, humidity, and pressure excursions that can compromise product quality.
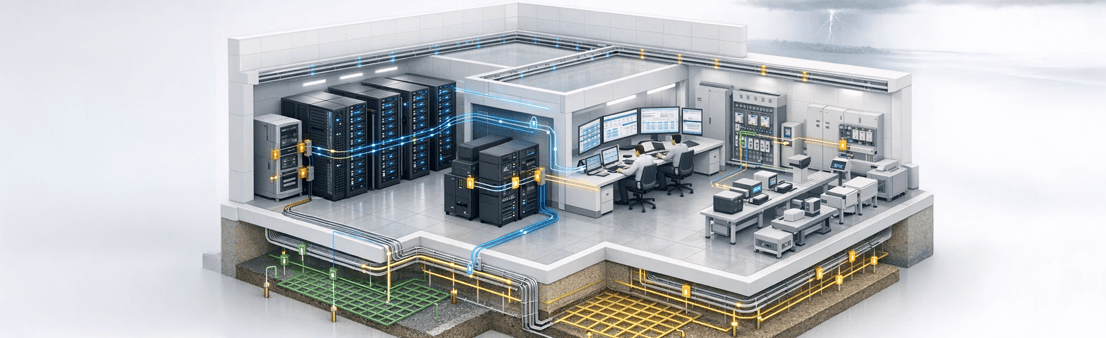
- ERP, MES, and LIMS servers
- PLC, SCADA, and BMS systems
- Batch records and serialization databases
- Weighing, barcode, and track-and-trace systems
- Type 3 SPDs at equipment level
- Data and signal line surge protectors
- Shielded cabling and equipotential grounding
- Dedicated IT earthing systems
- Surges impacting ERP, MES, LIMS, PLC, SCADA, and BMS
- Data loss in batch records, serialization, and audit trails
- Signal surges, EMI, and GPR causing system faults and downtime
These measures protect manpower databases, electronic batch records, audit trails, and regulatory data.
- QC and analytical laboratories
- Stability chambers
- R&D pilot plants
- Precision surge protection for sensitive instruments
- Structural lightning protection for laboratory buildings
- Equipment earthing and bonding
- Surges impacting ERP, MES, LIMS, PLC, SCADA, and BMS
- Data loss in batch records, serialization, and audit trails
- Signal surges, EMI, and GPR causing system faults and downtime
- Water for Injection (WFI) plants
- Purified water systems
- Effluent treatment plants (ETP)
- Compressed air and nitrogen systems
- Vaccine and biologics cold storage
- Lightning rods and air terminals
- Surge protection for motor panels
- Earthing of all metallic and utility structures
- Lightning & surges risk WFI, water, ETPs, and utility continuity
- Power overvoltage affects motors, compressors, air, nitrogen, and cold chains.
- GPR & poor earthing cause equipment damage and safety and compliance loss
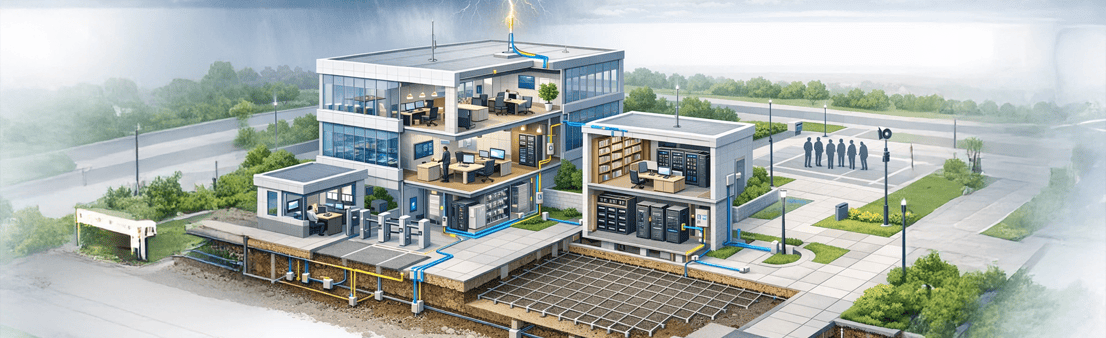
- Office buildings and documentation centers
- Security cabins and access control systems
- Emergency systems and assembly points
- Structural lightning protection systems
- Power and data SPDs
- Step and touch voltage control
- High occupancy increases human exposure
- Lightning and surge events pose shock risks
- Fire hazards threaten occupant safety
- System failures disrupt safe evacuation
Why LPES for Pharmaceutical Lightning Protection
- End-to-end lightning and surge protection under a single trusted brand
- Engineered for GMP compliance, data integrity, and auditreadiness
- Protects infrastructure, assets, production, digital systems, and human life
- Reduces downtime, batch loss, and compliance risk